Introduction: Chimeric antigen receptor (CAR) T cell therapy has markedly improved treatment outcomes for patients with relapsed/refractory B-cell malignancies. However, CAR-T cells are also associated with a distinct toxicity profile including cytokine release syndrome (CRS) and neurotoxicity (ICANS). Due to their nature as a ‘living drug’, CAR-T cells can also exert profound and long-lasting immune deficits - predisposing for a broad range of infectious complications. While there is a growing body of evidence concerning treatment response and relapse mechanisms, data on non-relapse mortality (NRM) remains scarce. To gain further insights into drivers of non-relapse related deaths after CAR-T therapy, we performed a meta-analysis of published clinical trials and real-world studies (RWS).
Methods: For our literature search of clinical trials, we included all studies which led to approval of the six commercially available CAR-T products (axi-cel, tisa-cel, ide-cel, cilta-cel, liso-cel, brexu-cel) and the corresponding phase I-II (Ph I/II) trials. For RWS, articles had to be published until June 2023 and had to fulfill the following criteria: (1) use of commercially available CAR products in a real-world setting, (2) inclusion of > 100 patients, (3) reports on adult patients. Studies which did not differentiate between relapse and non-relapse related deaths were excluded. Data extraction included basic study characteristics (patient numbers, follow-up time, CAR product), NRM, causes of death, and treatment-association.
Results: From a total number of 511 screened studies, 15 clinical trials (Ph I/II: 9, Ph III: 6) and 9 RWS compromising 5,313 patients fulfilled the inclusion criteria. As expected, median follow-up was longer for Ph III trials compared to RWS (Ph III: 18.0 (16.3-34.9) months, RWS: 12.9 (9.7-14.2) months, p=0.04), and comparable to Ph I/II trials (17.5 (15.9-28) months, p>0.9). Among all studies, the most common entity was large B-cell lymphoma (LBCL, 3,770 patients), followed by multiple myeloma (MM, 959), mantle cell lymphoma (MCL, 339) and indolent lymphomas (IL, 245). The distribution of encountered CAR products in the study cohort was: 10 axi-cel, 6 tisa-cel, 4 ide-cel, 4 liso-cel, 3 brexu-cel, and 2 cilta-cel.
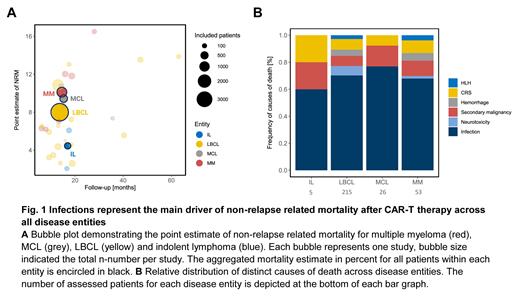
Of note, over half of the studies did not report any measure of NRM (58%), highlighting deficits in toxicity reporting among CAR-T cell studies. We observed considerable heterogeneity in reporting of NRM with follow-up times ranging from 3 to 12 months and NRM rates ranging between 3.0-9.1%. To integrate studies without a reported NRM, we calculated a point estimate of NRM by dividing non-relapse related deaths by the total number of respective patients ( Fig. 1A). Importantly, the comparison of reported NRMs and NRM point estimates was similar (p=0.89). We noted a trend towards higher NRM estimates in patients with MM (9.4% IQR 6.5-12%) followed by MCL (8.7% IQR 8.1-9.7%), LBCL (6.2% IQR 4.4-8.4%) and IL (4.1% IQR 3.1-5.1%, Kruskal-Wallis p=0.12). NRM was lowest with tisa-cel (3.4% IQR 2.8-4.3%) and highest with cilta-cel (14.3% IQR 13.1-15.4%, Kruskal-Wallis p=0.014). Numerically increased NRM was noted for CD28z- vs. 4-1BB harboring CAR products (8.2 vs. 6.2%, p=0.13) and for phase III studies (Ph I/II vs. III vs. RWS: 6.6 vs. 11.9 vs. 6.2%, Kruskal-Wallis p=0.16).
To further elucidate the etiology of non-relapse related deaths, we categorized all reported deaths into one of the following categories: CRS, neurotoxicity, infection, hemorrhage, secondary malignancy, HLH, organ failure not otherwise specified (NOS), others/unknown. In total, 441 deaths were reported of which nearly half were caused by infections (n=210, 47.6%). The CAR-T specific adverse events CRS, ICANS, or HLH led to death in 11.1% of cases. Of interest, secondary malignancies contributed to death in 6.1% of cases and hemorrhages in 3% of cases. We observed a similar distribution of CAR-specific toxicities across entities ( Fig. 1B chi 2 p=0.78).
Conclusions: Infections were by far the main determinant of non-relapse mortality across all disease entities, underlining the profound immune deficits conferred during CAR-T therapy. The high incidence of secondary malignancies of 6% is concerning and warrants further systematic study. High-quality and long-term reporting of NRM is critical to recognize emerging side effects and to identify potential toxicity signals of novel CAR products.
Disclosures
Rejeski:BMS/CELGENE: Consultancy, Honoraria; Novartis: Honoraria; Kite/Gilead: Other: Travel Support, Research Funding; Pierre-Fabre: Other: Travel Support.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal